What Makes Ammonium Iodide an Ideal Release Agent?
Ammonium iodide is the scientific name of the chemical compound NH₄I. It is used in colour dyes, explosives, photographic chemicals, medications and fire extinguishers, and is created by combining ammonia and hydroiodic acid.
How to Prepare Samples for XRF Analysis
XRF analysis is a comparative chemical analysis technique that can precisely identify and quantify myriad different elements in samples. These are identified under an X-ray by using flux. Flux shows the intensity of an X-ray beam, as well as the number of photons that appear and the per unit time from the X-ray. The measurement for flux is defined as photons per second, also known as p.p.s.
There are many different methods that can be used for sample preparation when it comes to xrf analysis. These include:
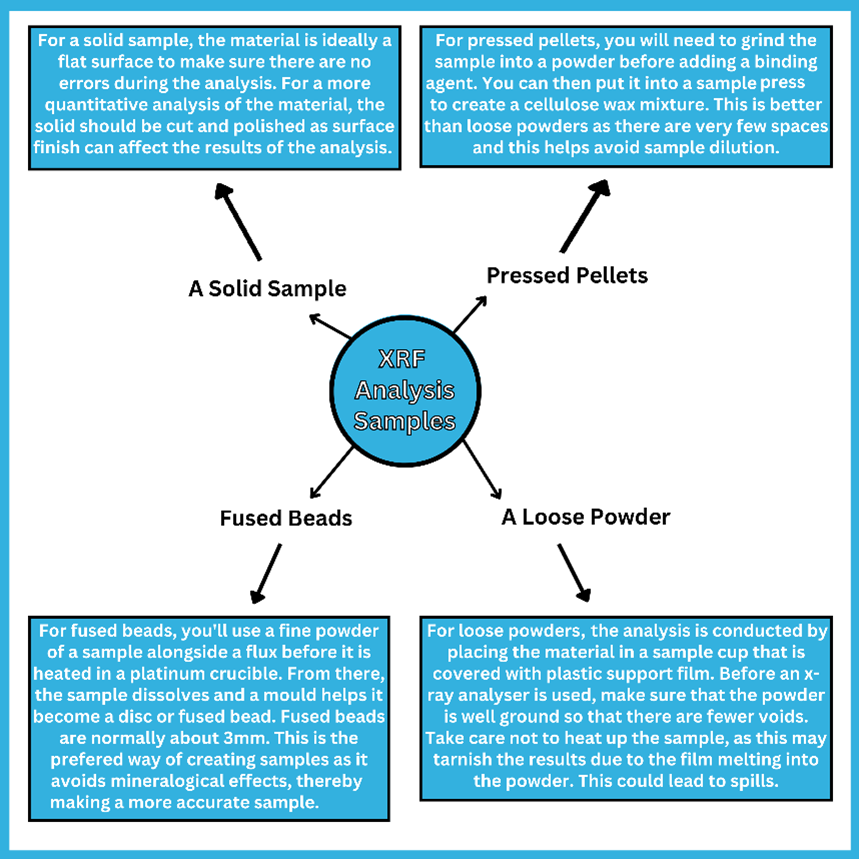
As stated previously, fused beads tend to be the best way to create a sample. If you want to accurately analyse a sample by using XRF flux, you’ll find that they tend to be fused with eutectic mixtures to create a glass bead or disc. However, there can be problems with these beads, as sometimes the fusion mixture can stick to the crucible whilst pouring into the mould. This is where a release agent can be used to free it.
With that said, there are a few release agents you can look at that to help with the XRF flux process, including ammonium iodide.

The Benefits of Ammonium Iodide
Ammonium iodide makes a great release agent for any type of melt because the surface tension will be increased. Ultimately, this will help lower the bead’s surface area that touches the platinum crucible. Therefore, you will find it easier to release the fluid melt from the crucible. As a result, there will be a lower amount of hang-up from the melt. It will also make it so much simpler to clean any equipment that you use during the testing process, including the crucible itself.
To summarise, some of the benefits of using ammonium iodide tablets include:
- Less damage to your platinum crucible or mould because it is less likely that you will forcibly remove a bead during the release process
- The process providing better performance results because the bead’s surface will be flatter and more consistent
- The crucible and equipment becoming easier to clean after release agents
- A higher success rate and amount thanks to the fluidity of the release process
Why is Ammonium Iodide a Better Release Agent than Bromides?
Xrf flux is great for screening raw materials, however you will need to use a release agent if you want to free a fused bead from its crucible. It’s wiser to use ammonium iodide tablets than bromides. This is because the Br LA actually inhibits the AI Ka, making it harder to get an accurate and successful bead after flux or sample preparation.
Another thing that makes ammonium iodide a better release agent than bromides is the formed bead’s positive or neutral meniscus. By using this release agent, it will be easier to reduce the likelihood of a bead fracture or a bead being created with a sharp edge. This, in turn, lowers the possibility of a reading error.
Are you Ready to Purchase Ammonium Iodide as a Release Agent?
Ammonium iodide tablets make excellent release agents and come with a wide variety of benefits. Iodine compounds can evaporate quickly at lower temperatures and can make it easier to clean crucibles as they have fewer cleaning requirements. If you need a release agent to defuse your beads or discs, you should use ammonium iodide.
To find out more about release agents in relation to xrf flux, speak to XRF Scientific today! They form ammonium iodide tablets under controlled conditions. This will ensure their performance, consistency and quality. Use our contact form to get in touch with product specialists today.









